Quantitative determination of carbonate and bicarbonate in water
The kids took 25 ml samples of bottled and tap water in Erlenmeyer flasks. Then, they added five drops of phenolphthalein and checked whether they could notice some pink color, which would mean that there were carbonates in water. To find out the miligrams per litre of carbonate they would have to pour drops of 0,1 M HCl(aq) from a burette in the flasks until the pink color disappeared. We can calculate the mg/L of carbonate after the spent volume of HCl(aq), as the moles of carbonate are the same as the reacted moles of HCl.
The test went on by adding five drops of methyl orange and titrating with HCl(aq) until the color of the dissolution changed from orange to red. The moles of bicarbonate measured this way include those that previously were in the form of carbonate, so they have to be substracted in order to find out the moles of bicarbonate in the sample of water.
Bottled water
No pink color was observed after adding phenolphthalein, so no carbonate was detected. The results of the titration with 0,1 M HCl(aq) and methyl orange dye are as follows
Bicarbonate
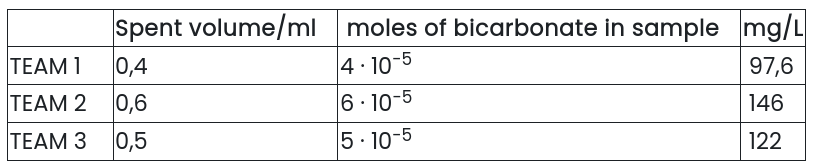
Average: 122 mg/L
In the label of the bottle can be read 108 mg/L

